Question
- Leaving Certificate Chemistry (Higher) 2022: Section B Q6
- Back to the question >
Answer
(a)
(i) Petrol fractions have 5-10 carbons per molecule and so have a smaller average molecular mass than the diesel fraction, which contains 14-19 carbons per molecule.
(ii) The diesel fraction has a larger boiling point range (525-625 K) than the petrol fraction (300-425 K).
(b)
(i) The octane number of a fuel is the measure of the tendency of a fuel to resist auto-ignition or knocking.
(ii) Heptane
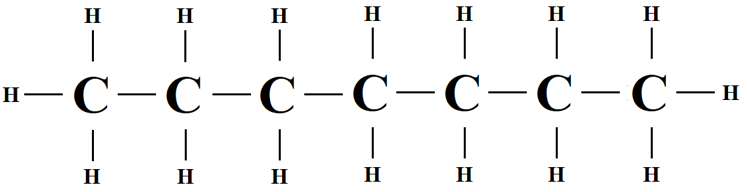
(iii) 2,2,4-trimethylpentane
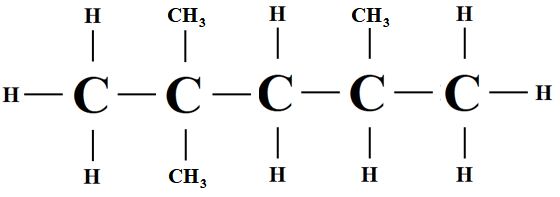
(iv) Short chain and high degree of branching.
(c)
(i) Hydrogen gas does not occur in nature because it is very reactive so it is normally found as part of chemical compounds.
(ii) Electrolysis of water.
(iii) Heat of reaction is the heat in kilojoules released or absorbed when the number of moles of the reactants indicated in a balanced equation reacts completely.
(iv) The heat change of a reaction is independent of the route by which the reaction may occur (Hess’ law).
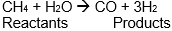
Heat change = products - reactants
206.5 = (-110.5 + 0) — (CH4 + (-241.8))
206.5 + 110.5 - 241.8 = -CH4
75.2 = -CH4
CH4 = -75.2 kJ/mol
