Question
- Leaving Certificate Chemistry (Higher) 2022: Section B Q8
- Back to the question >
Answer
(a)
(i) Carboxylic acid functional group.
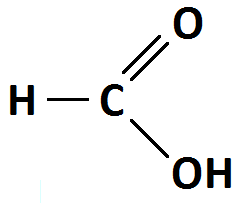
The carboxylic acid is acidic in nature because of the hydrogen in the COOH group. This allows carboxylic acids to dissociate in aqueous solutions forming hydrogen gas, due to the donation of its hydroxyl proton.
(ii) The reaction between ethanol and sodium metal.
CH3CH2OH + Na --> CH3CH2ONa + ½ H2
(iii) There is no reaction between ethanol and sodium carbonate because ethanol is an extremely weak acid with very little tendency to donate protons in a reaction with sodium carbonate.
(b)
(i)

(ii) E: Ethyl methanoate
(iii) 1 planar carbon
(iv) Substitution reaction
(v) F: Methyl ethanoate
(vi)
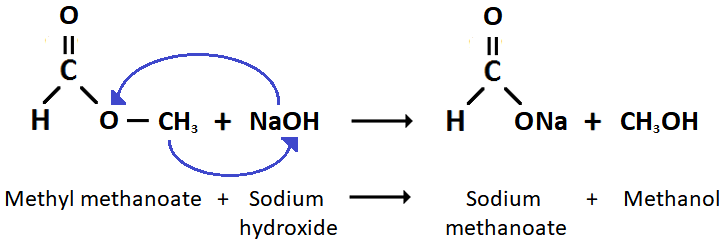
(vii) The boiling point of carboxylic acids is higher because there is intermolecular hydrogen bonding between the ethanoic acid molecules but this does not occur between the ester molecules.
