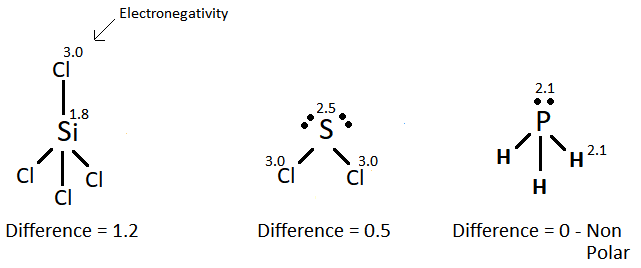Question
- Leaving Certificate Chemistry (Higher) 2022: Section B Q10
- Back to the question >
Answer
(b) Electronegativity is the measure of pulling power that atoms have for a shared pair of electrons in a covalent bond.
As you move down group 17 in the periodic table the electronegativity decreases because:
- The atomic radius increases; therefore, there is less pull on the outer electrons by the nuclear charge.
- The number of shells increases causing screening; therefore, there is less pull on the outer electrons by the nuclear charge.
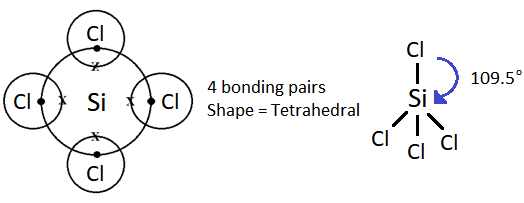
(i) Shape is tetrahedral.
SiCl4
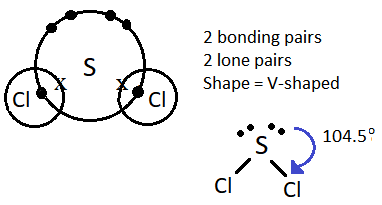
(ii) Shape is v-shaped
SCl2
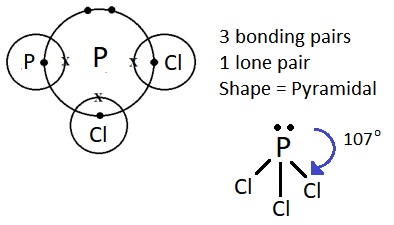
(iii) Shape is pyramidal
PCl3
(iv) Silicon tetrachloride
(v) Sulfur dichloride has bonds with the least degree of polarity.