Question
- Leaving Cert. Chemistry (Higher) 2016: Section B Q11
- Back to the question >
Answer
Arsine has pure covalent bonding.
Dot and cross diagram of bonding in arsine:
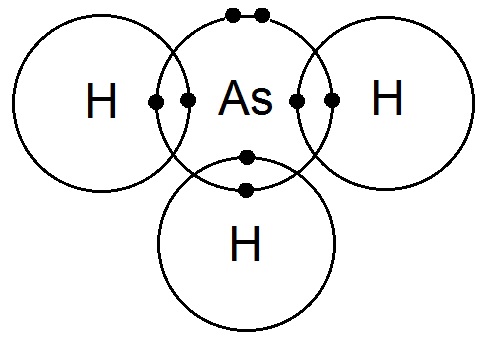
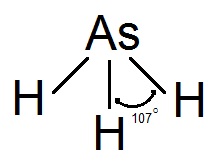
Arsine has 3 bonding pairs and 1 lone pair of electrons so the shape of the molecule is pyramidal.
NH3 is the only hydride that will have intermolecular hydrogen bonding because this intermolecular force only occurs between molecules that contain a hydrogen, which is covalently bonded to nitrogen, oxygen or fluorine.
(i)
Ammonia has the highest boiling point of the three hydrides because of the hydrogen bonding between the molecules, which is very strong and so requires more energy to break.
(ii)
Phosphine is a smaller and lighter molecule than arsine, so less energy is required to break the bonds between the molecules; therefore, it has a lower boiling point.
