Question
- Junior Cert. Science SEC Sample: Q11
- Back to the question >
Answer
(a)
Method:
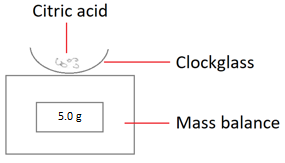
1. Measure out 5 g of citric acid using a mass balance.
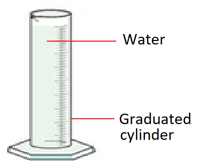
2. Measure 100 ml of water using a graduated cylinder.
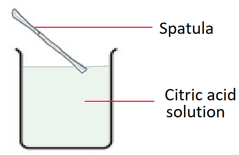
3. Transfer water into a clean beaker and add the citric acid to the water. Stir to dissolve.
(b)
This is an endothermic reaction where heat energy is absorbed by the reactants during a chemical reaction.
This is an endothermic reaction where heat energy is absorbed by the reactants during a chemical reaction.
(c)
Baking soda is a base so when it is added to the citric acid it will cause the pH of the solution to increase towards neutral.
Baking soda is a base so when it is added to the citric acid it will cause the pH of the solution to increase towards neutral.
(d)
To investigate how pH changes during the reaction:
To investigate how pH changes during the reaction:
- Add a few drops of universal indicator to the citric acid solution.
- Note the colour of universal indicator and find the pH using the pH colour chart.
- Add baking soda to the citric acid solution.
- Note colour change of universal indicator and find the pH using the pH colour chart.
