Question
- Leaving Cert. Chemistry (Higher) 2019: Section B Q7
- Back to the question >
Answer
(a)
(i) HCl + H2O -----> Cl- + H3O
(ii) NH3 + H2O -----> NH4+ + OH-
(iii) The stronger an acid, the weaker its conjugate base. Cl- is a poor proton acceptor.
(iv) The weaker the base, the stronger its conjugate acid. NH4+ is a good proton donor.
(b)
(i) Kw = [H3O+] x [OH-]
(ii) The value of Kw increases as the temperature increases; therefore, the equilibrium moves forward, indicating an endothermic reaction (energy absorbed to break bonds).
(iii)
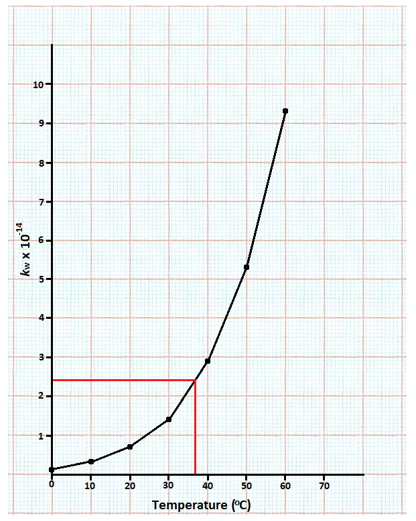
(iv) At 37o Kw = 2.4 x 1014
(v)
Kw = [H3O][OH-] = 2.4 x 10-14
[H3O] = [OH-]
[H3O]2 = 2.4 x 10-14
[H3O] = √2.4 x 10-14
[H3O] = 1.549 x 10-7
(vi)
pH = −log10 [ H3O+]
6.77 = −log10 [H3O+]
Antilog −6.77 = [H3O+]
1.698 x 10-7 = [H3O+]
[H3O+] = [OH-]
Kw = [H3O +][ OH-]
Kw = 1.698 x 10-7 x 1.698 x 10-7
Kw = 2.883 x 10-14
Temperature = 40 oC
