Question
- Leaving Cert. Chemistry (Higher) 2019: Section B Q8
- Back to the question >
Answer
(a)
(i) Secondary alcohols have two carbons attached to the carbon with the hydroxyl group (-OH).
(ii)
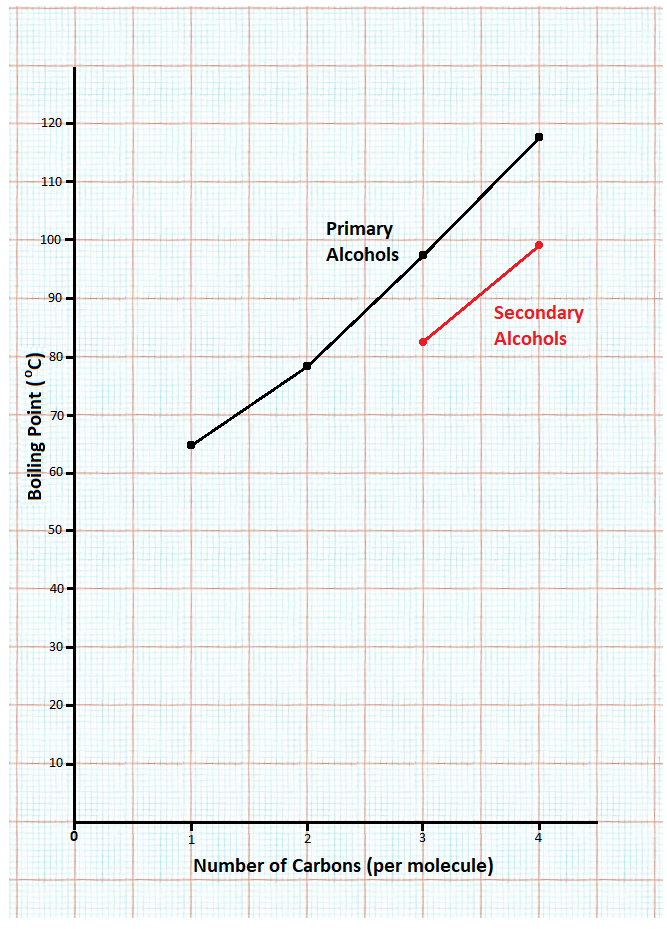
(iii) The boiling points of the four primary alcohols increase as their molecular mass increases as there is hydrogen bonding between the molecules, which is a strong intermolecular bond. Therefore, the larger the molecule, the more energy required to break these bonds; hence, a higher boiling point.
(iv) Approximate boiling point: 118-119 oC
(b)
(i) Butanal and butanoic acid
(ii)
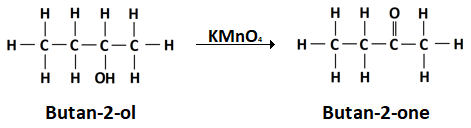
(iii) C-O and O-H
(c)
(i)
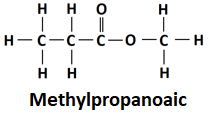
(ii) 3 carbons are tetrahedrally bonded.
(iii) Products: Sodium propanoate and methanol
