Question
- Leaving Certificate Chemistry (Higher) 2020: Section A Q3
- Back to the question >
Answer
(a)
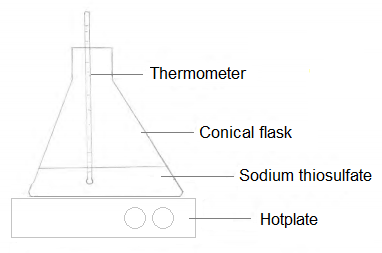
The conical flasks were heated using a hot plate. The temperature was measured using a thermometer.
(b)
(i) A creamy white precipitate of sulfur was produced.
(ii) When the cross on the paper is no longer visible due to this precipitate, the stop clock was stopped immediately.
(c)
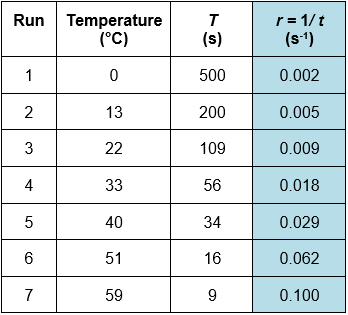
(d)
(i)
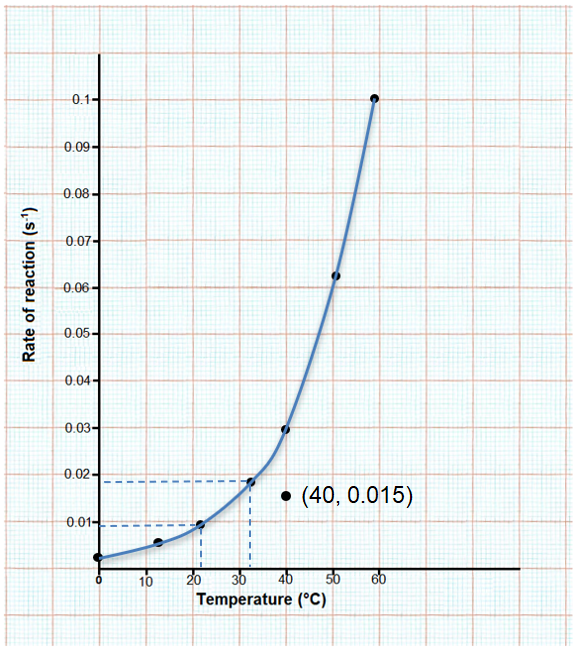
(ii) The rate of this reaction doubles for every 10o C rise in temperature.
(iii) At 22oC the rate of reaction = 0.009 and at 33oC the rate of reaction = 0.018. Therefore, an increase of 10oC causes the rate of reaction to double.
(e)
(i) (40, 0.015) does not lie on the curve that is plotted.
(ii)
1 / t = 0.015 s-1
t = 1 / 0.015 = 66.6 s
Run 5 at 40oC = 34 s and rate = 0.029 s-1
There was an increase in time for Run 8 and therefore, a decrease in rate of reaction so the depth of liquid was less than the original flasks.
