Question
- Leaving Certificate Chemistry (Higher) 2020: Section B Q9
- Back to the question >
Answer
(a) Chemical equilibrium is the state in which the rate of the forward reaction equals the rate of the reverse reaction.
Chemical equilibrium is said to be dynamic because at equilibrium there are reactions continuously occurring.
Le Châtelier’s principle states that when a system at equilibrium is subjected to a stress, the equilibrium shifts in such a way as to minimise the effect of the stress.
(b) Kc = [CO2][ H2] / [CO] [H2O]
(c)
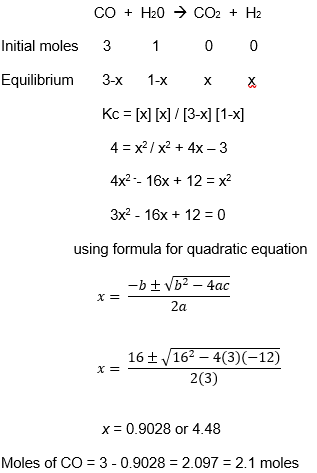
(d)
(i) There will be no effect as the change in concentration will not change Kc as it is only affected by a change in temperature.
(ii) The value of hydrogen will increase as the addition of steam will favour the forward reaction.
(e) The value of Kc will decrease as the temperature is increased because the forward reaction is exothermic.
