Question
- Leaving Certificate Chemistry (Higher) 2021: Section B Q10
- Back to the question >
Answer
(a)
(i) Cross and dot diagram:
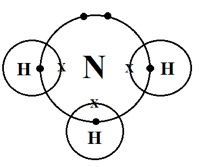
(ii) NH3 molecules contain a lone pair of electrons.
(iii) Shapes: Linear and V-shaped
(iv)
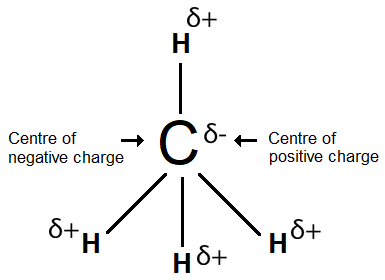
In the CH4 molecule the centre of the positive charge will be at the geometric centre of the molecule so it coincides with the centre of negative charge; therefore, this molecule is non-polar.
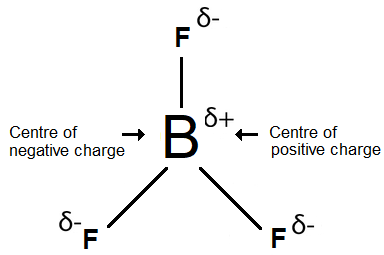
In the BF3 molecule the centre of the negative charge is at the geometric centre of the molecule so it coincides with the centre of positive charge; therefore, this molecule is non-polar.
(v) The bond angle for NH3 (107.0) is less than the bond angle for CH4 (109.5). The presence of the lone pair on the NH3 molecule causes the bonding pairs to be pushed closer together and this causes a decrease in the bond angle.
